
CHEAP
GASOLINE ! ![]()
A Zero-Emissions Synthetic Oil
Refinery
making Net-Zero Emissions Coal + Biomass Transportation Fuel
Peak Oil and Global Warming
http://www.fischer-tropsch.org/
Severe search blocking
http://www.covol.com/coalLiquids.asp
Headwaters
http://www.americanfuelscoalition.com/
http://www.rentechinc.com/processFAQ.php GTL
http://en.wikipedia.org/wiki/Sulfur-iodine_cycle
http://www.netl.doe.gov/technologies/hydrogen_clean_fuels/refshelf/refshelf.html
http://en.wikipedia.org/wiki/Karrick_process Using reactor or plasma
torch heat may give this a Karrick-like process.
Contents
of this page
1)
Introduction
2) Two
studies on future coal-to-liquids refinery growth.
3)
Synthetic BioGasoline from
Blends of U.S. Coal +
Biomass
4) A
Zero-Emissions Coal + Biomass Synthetic Oil Refinery
5) The coal-to-liquids
technologies
6)
Coal + Biomass Zero-Emissions Synthetic Oil
Refinery Diagrams
7)
8)
1) Introduction
This pushes coal-to-liquids refinery technology beyond its current frontiers. There has never been a nuclear powered, zero emissions, coal + biomass coal-to-liquid refinery before. In writing about one, the author earns several chutzpah awards. The author has no formal background in either 1) nuclear energy or 2) CTL refineries. This is almost Jules Verne territory. But the thorium-fueled, molten salt reactor has been built, tested, and well-documented by Oak Ridge National Laboratories and Sundrop Fuel's state-of-the-art-clean natural gas + biomass refinery is springing up near Alexandria, Louisiana.
2) Two studies on future coal-to-liquids refinery growth.
Our government has a path to U.S. Energy security and independence. For example, we will have to pump about 9 million barrels per day in 2007. In fact, we actually pumped less than 5. The projection below says we will be pumping less than 2 million barrels per day by 2030 compared to the 7 shown at right.

(Right) British Petroleum's take on global Non-Conventional oil sources up to 2020.
3) Synthetic BioGasoline from Blends of U.S. Coal + Biomass
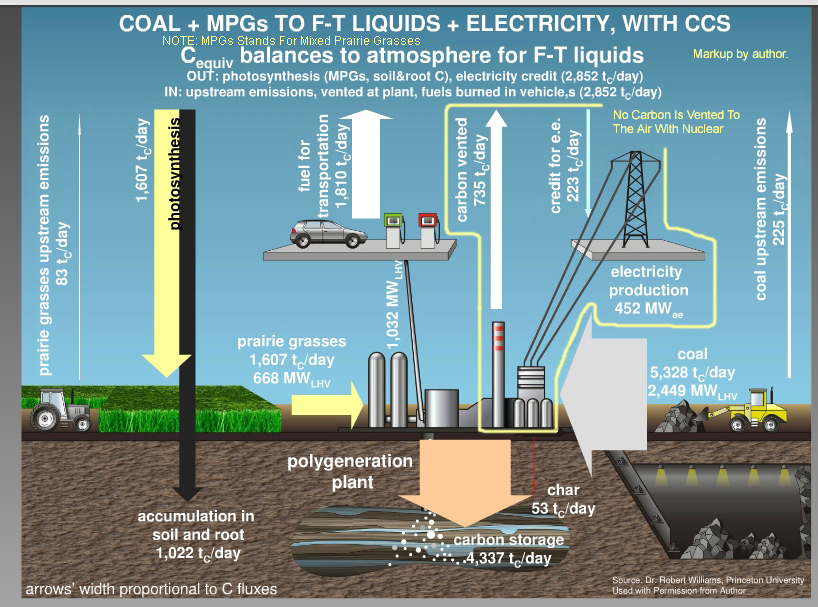
4) A Zero-Emissions Coal + Biomass Synthetic Oil Refinery (From Summary Page)
A Zero-Emissions Synthetic Oil
Refinery for Making Net-Zero Emissions Transportation Fuels
This is the difficult part. By using high temperature nuclear heat instead of coal heat to make coal-to-oil, you are painting with a different palette.
The RAND Corporation studies indicate that complete capture and disposal of CO2 emissions would add less than $5 to a barrel of CTL oil, burning coal to make oil.
Co-generation of electricity by burning unusable process gas is the major source of CTL's Global Warming emissions. Since this is no longer acceptable, this gas must be kept inside the product stream or become part of the CCS disposal stream.
If coal-to-oil is to be done squeaky-clean, we will
have to use high temperature nuclear heat and capture the inevitable streams of CO2
and H2S.
This will take more processing, more expensive
heating equipment
such as
Westinghouse-plasma electric torches with 1,000 hour electrode life to
vaporize the coal feedstock. ![]() But it can be done. The
author has a 2002 Westinghouse Plasma Corporation paper about a
"next-generation" Plasma Gasification Reactor study with a capacity of 360
tons/day. 42 of these gassifiers would vaporize the 15,000 tons of coal per day.
At 1/2 billion dollars.
But it can be done. The
author has a 2002 Westinghouse Plasma Corporation paper about a
"next-generation" Plasma Gasification Reactor study with a capacity of 360
tons/day. 42 of these gassifiers would vaporize the 15,000 tons of coal per day.
At 1/2 billion dollars.

The biggest hit will be the additional energy needed to make clean happen. But, heat from thorium is so cheap it is almost free. That's the trade-off.
And nuclear promises even cleaner, more energetic synthetic fuels (higher miles-per-gallon) than today's fuels if we can manage to cleanly obtain the hydrogen available from water to upgrade the oil molecule even more. "A nuclear source of hydrogen coupled with nuclear process heat would more than double the amount of liquid hydrocarbons from the coal and eliminate most CO
2 emissions from the process." - http://world-nuclear.org/info/inf116_processheat.html
The coal-to-oil conversion process produces
ultra-clean clean fuels with all of coal's solid pollutants being trapped
in the coal's solid waste char and gas pollutants captured during refining. Coal-to-oil conversion is a well-known process, and
its environmental aspects are well-documented by many sources.
(Click to enlarge.)
![]()
Environmental impact. Converting our 286 largest power plants from coal to nuclear to make both electricity and synthetic oil would end about 40% of ALL U.S. Global Warming emissions. If the solid waste, CO2, and H2S (sulfur) from the coal refining process is sequestered, then there should be no objections about use of ultra-clean synfuels produced from coal.
Replacing all of America's imported oil would consume a little more than ALL the coal the U.S. is currently burning to make electricity.
To make this all environmentally sane, the EPA should make the allowable combined power plant and coal-to-oil refinery emissions equal to or lower than had the power plant alone converted to "Carbon Capture and Sequestration" emissions control instead, i.e., an 80% or more reduction in ALL emissions, including CO2 (carbon dioxide). CCS backgrounder: http://en.wikipedia.org/wiki/Carbon_capture_and_storage
More on the zero-emissions synthetic oil refinery page >
"The new process could cut the energy cost of producing the fuel by 20 percent just by rejiggering the intermediate chemical steps, said co-author Ben Glasser of the University of the Witwatersrand in Johannesburg, South Africa. But coal-derived fuel could produce as much as twice as much CO2 as traditional petroleum fuels and at best will emit at least as much of the greenhouse gas.
Glasser’s new production method allows them to set a lower limit on the amount of energy that would be needed to transform solid coal into fuel. The very best possible CTL process would require 350 megawatts of input to make 80,000 gallons of fuel; the current process uses more than 1,000 megawatts."
Please read the entire article at: http://www.wired.com/wiredscience/2009/03/coaltoliquids/
This is the difficult part. By using high temperature nuclear heat instead of coal heat to make coal-to-oil, you are painting with a different palette. All technologies will have to be checked out - FT CTL, GTL, and MTG seem to be favored for controllable emissions and product market compatibility but not efficiency. Against this backdrop, we have Shenhua, a direct conversion process and the largest to be built since South Africa. Headwaters is known to have participated in this effort.
Key to coming clean is unlimited amounts of clean hydrogen. Brute force electricity from a local generator comes to mind.
Burning coal to convert coal-to-oil (CTL) is very environmentally dirty and will probably never be considered outside of China or India. Pre-nuclear coal-to-oil refinery technology is optimized for economic yield rather than emissions at about 50%, i.e., one ton of bituminous coal yields two barrels of oil. It is unreasonable to expect non-nuclear coal-to-oil refineries to be totally environmentally clean or to make frugal use of the feedstock. While the Chinese have made some progress cleaning up their new coal powered coal-to-oil process at Shenhua, no one has ever tried to engineer a nuclear powered "ultra-clean, don't spare the energy" coal-to-oil refinery.
The RAND Corporation studies indicate that complete capture and disposal of CO2 emissions would add less than $5 to a barrel of CTL oil, burning coal to make oil.
Co-generation of electricity by burning unusable process gas is the major source of CTL's Global Warming emissions. Since this is no longer acceptable, this gas must be kept inside the product stream or become part of the CCS disposal stream.
The energy challenges a FT CTL plant presents are substantial, even for a 1,300°F liquid thorium reactor. Pyrolyzation temperatures range from 1,800°F to 2,700°F at 500 psi, substantial amounts of heat must be extracted - some of which can be used to produce electricity or feedstock drying - and substantial amounts of hydrogen may be needed for fuel upgrading.
A Bergius direct liquefaction plant calls for 750°F,
1,500 psi, and huge amounts of hydrogen.
http://en.wikipedia.org/wiki/Sulfur-iodine_cycle (1,560°F)
http://www.chinatungsten.com/Molybdenum/Molybdenum-Disilicide-heating-elements.html
http://www.sentrotech.com/mosi2-molybdenum-disilicide-heating-element
http://andy666.en.ec21.com/1900C_Molybdenum_Disilicide_Heating_Elements--2258650.html
http://keithcompany.com/clientfiles/MoSiO2%20Data.pdf
If coal-to-oil is to be done squeaky-clean, we will
have to use high temperature nuclear heat and capture the inevitable streams of CO2
and H2S.
This will take more processing, more expensive
heating equipment
such as
Westinghouse-plasma electric torches with 1,000 hour electrode life to
vaporize the coal feedstock. ![]() But it can be done. The
author has a 2002 Westinghouse Plasma Corporation paper about a
"next-generation" Plasma Gasification Reactor study with a capacity of 360
tons/day. 42 of these gassifiers would vaporize the 15,000 tons of coal per day.
At 1/2 billion dollars.
But it can be done. The
author has a 2002 Westinghouse Plasma Corporation paper about a
"next-generation" Plasma Gasification Reactor study with a capacity of 360
tons/day. 42 of these gassifiers would vaporize the 15,000 tons of coal per day.
At 1/2 billion dollars.
The biggest hit will be the additional energy needed to make clean happen. But, heat from thorium is so cheap it is almost free. That's the trade-off.
And nuclear promises even cleaner, more energetic synthetic fuels (higher miles-per-gallon) than today's fuels if we can manage to cleanly obtain the hydrogen available from water to upgrade the oil molecule even more. "A nuclear source of hydrogen coupled with nuclear process heat would more than double the amount of liquid hydrocarbons from the coal and eliminate most CO
2 emissions from the process." - http://world-nuclear.org/info/inf116_processheat.html
The coal-to-oil conversion process produces
ultra-clean clean fuels with all of coal's solid pollutants being trapped
in the coal's solid waste char and gas pollutants captured during refining. Coal-to-oil conversion is a well-known process, and
its environmental aspects are well-documented by many sources.
(Click to enlarge.)
![]()
Environmental impact. Converting our 286 largest power plants from coal to nuclear to make both electricity and synthetic oil would end about 40% of ALL U.S. Global Warming emissions. If the solid waste, CO2, and H2S (sulfur) from the coal refining process is sequestered, then there should be no objections about use of ultra-clean synfuels produced from coal.
Replacing all of America's imported oil would consume a little more than ALL the coal the U.S. is currently burning to make electricity. A similar process using natural gas as "Gas-to-Liquids" (GTL) feedstock is being used now by Shell in Qatar. Shell's Pearl There is enough American natural gas to replace three times the oil America imports from the Mideast. A good learning example is the " Mini-GTL ". With combinations of both coal and natural gas (not to forget our oil sands and oil shale), America can supply all of America's oil needs - even when all pump-able oil is gone.
Similar "unconventional oil-to-liquid oil" processes powered by the vast amounts of cheap and clean high temperature heat available from liquid thorium can also be used for oil sands, shale oil, and oil sludge deposits so we will never, ever, run out of oil. Along these same lines of thinking, given enough cheap electricity, plasma torches can also gasify solid municipal waste - what your garbage truck picks up - into fuel feedstock, metal, and glass-like waste ash.
To make this all environmentally sane, the EPA should make the allowable combined power plant and coal-to-oil refinery emissions equal to or lower than had the power plant alone converted to "Carbon Capture and Sequestration" emissions control instead, i.e., an 80% or more reduction in ALL emissions, including CO2 (carbon dioxide). CCS backgrounder: http://en.wikipedia.org/wiki/Carbon_capture_and_storage
5) The coal-to-liquids technologies
The Technologies
Direct
Conversion
Based on high-pressure dissolution of coal
More energy efficient than indirect liquefaction
Produces high energy density fuels
- Diesel fuel low cetane #
- High aromatics
Used by Germany in WWII, improved by U.S., now being deployed in China
Advantages
Conceptually simple process
Produces high-octane gasoline
More energy efficient than indirect conversion (i.e., more fuel / BTUs produced
per ton of coal)
Products have higher energy density (BTU/gallon) than indirect conversion
Disadvantages
High aromatic content
Low-cetane number diesel
Potential water and air emissions issues
Fuels produced are not a good environmental fit for the U.S. market
May have higher operating expenses than indirect conversion
Indirect
Conversion (This technology appears
to the author to be most promising from an environmental standpoint.)
Based on gasification
Converts syngas (H2
and CO) into clean methanol or hydrocarbon liquids
Can also produce ultra-clean diesel or jet fuel
CO2 can be
captured for sequestration
Can co-produce electric power or hydrogen
Advantages
Ultra-clean products
Well suited for CO2
capture
Well suited for electric power co-production
May have lower operating expenses than direct conversion
Disadvantages
Conceptually more complex than direct conversion
Less efficient fuel production than direct conversion
Produces low-octane gasoline
Fewer BTUs per gallon than direct conversion products
Gasification of coal
Molten salts have also been studied since the early 1900s to gasify coal in a process called Molten Salt Oxidation (MSO). The molten salt used is usually sodium carbonate heated above its melting point of 851° C ( 1564° F) to around 900° - 1000° C. At this temperature the red hot salt functions as a catalyst, fluid reacting bed, and heat transfer medium; all in one! The coal is flash pyrolyzed such that no tars or oils are produced. Steam is usually injected too so that the combination of coal's thermally decomposed higher organic molecules along with catalytically assisted carbon-steam reactions (i.e., C + H2O = CO + H2) produces mainly carbon monoxide (CO) and hydrogen (H2) gases at atmospheric pressures. At higher pressures, there will be significant methane (CH4) and higher hydrocarbons produced. Carbon monoxide (CO) and hydrogen (H2) can be used directly as a fuel gas or as a synthesis gas to produce virtually any organic material. The most common use of synthesis gas however, is to produce Methanol (methyl alcohol - CH3OH) which can also be used as a fuel, and is used in race cars, but is usually a raw material for the production of various organics (octane boosters, gasoline additives, plastics, chemicals, and drugs).
Given the renewed interest in the so called "Hydrogen Economy", whereby hydrogen is used as a "carrier fuel" to provide energy portability and transmission, it is likely MSO will play a significant role in the production of hydrogen fuel for the Hydrogen Economy via the "water shift" reaction where the Synthesis gas (the mixture of CO and H2 gases described above) is converted into nearly pure H2 gas by the following, catalyzed reaction:
CO + H2O = CO2 + H2
The CO2 can then be fairly easily removed via various reactions, as it is a mild acidic gas which can be combined with various alkaline substances [e.g., CaCO3 (s) + H2O + CO2 = Ca(H2CO3)2 (aq)]. Generally, the more alkaline the substance the faster and more complete the reaction (absorption) of the CO2. Thus, sodium hydroxide (i.e., "Drano", or Lye - NaOH) would work much better at absorbing the carbon dioxide. Another method to remove the CO2 from the H2 gas is via liquefaction (using pressure and lower temperatures) of the carbon dioxide gas which liquefies much easier than the hydrogen gas. The liquefied CO2 could then be resold for various industrial or food processes.
Molten salt gasification of coal has also been
proposed for wastes, to include
garbage (Municipal Solid Wastes - MSW).
CTL - Molten Salt
Waste Oxidation .pdf
In the mid-1950s Rockwell, Inc. conducted
extensive tests of molten salts for the purpose of destroying chemical weapons.
This was also called Molten Salt Oxidation (MSO --> good description at Lawrence
Livermore National Lab's
Upadhye's MSO description), and was a spin-off of the earlier coal
gasification studies. Similar to the advantages of using molten sodium carbonate
to gasify coal, MSO has the additional advantage of having large amounts of
sodium in close proximity to the decomposing chemical weapons molecules. This is
significant because chemical weapons usually contain large amounts of fluorine,
sulfur and/or chlorine, all of which can form radicals which may cause the
production of carcinogens such as dioxins. The long residence times of the
chemical weapons in a molten salt bath, as compared to incineration, combined
with the presence of large amounts of sodium allows the chlorine, sulfur, and
fluorine radicals plenty of time to form stable, and safe, sodium compounds such
as sodium sulfate (a laundry soap and food additive), sodium chloride (table
salt), and sodium fluoride (an anti-cavity toothpaste ingredient). Although
there were no significant technical obstacles to employing MSO for chemical
weapons' destruction, widespread Molten Salt ignorance and inertia prevented its
deployment.
CTL
- Molten Salt Explosives Oxidation - Upadhye .pdf
6) Coal + Biomass Zero-Emissions Synthetic Oil Refinery Diagrams


The original of the above modified schematic came from:
